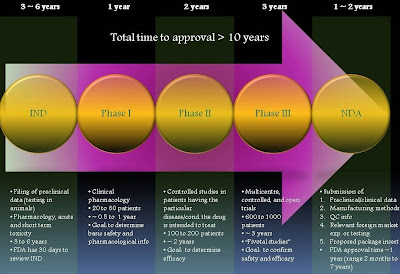
How do drugs get approved by the FDA?
After all, anxious investors will have to wait one more day for the results of the FDA's decision on the once-weekly version of the first-in-class Type 2 diabetes drug, Byetta, previously sold as a twice-daily injection. The probable scenario was that the FDA did not respond to the companies (Amylin (AMLN), Lilly (LLY), Alkermes (ALKS)) until late on Friday (Mar. 12,2010), and therefore, it was in the best interests of the drug makers to contain their excitement (or disappointment) and make the big announcement first thing in the following week to generate more buzz around the expected approval. Gaining a drug approval is a TREMENDOUS feat. This would only be fully understood if one were aware of the immense hurdles and challenges a drug/medical device candidate need to surpass in order to become commercially available. For novel biotech/pharmaceutical investors who are less clear on the process of advancing bench discoveries to bedside care, I thought I would take the opportunity to briefly summarize this road to glory for you.

No comments:
Post a Comment