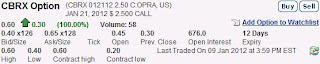
"Shares of Columbia Laboratories plunged more than 55% after FDA advisors voted against recommending the company's experimental drug for approval"
Columbia Laboratories Inc. and Watson Pharmaceuticals Inc. didn’t provide sufficient data that their progesterone gel reduces the risk of preterm birth, advisers to U.S. regulators said.
A Food and Drug Administration advisory panel voted 13-4 today in Silver Spring, Maryland, that the companies didn’t adequately prove the gel prevents mid-trimester births. The agency is scheduled to decide whether to approve the gel by Feb. 26 (PDUFA) and isn’t required to follow the panel’s recommendations.
Source: Bloomberg