Please note that the URL of this site will be changed from www.goldbiopharma.blogspot.com to www.michaeljuan.blogspot.com. Hopefully this will help you remember the site better and make the typing a bit easier! For those of you who have saved this blog as your bookmark, please don't forget to update the URL. The switch over will take place 2 weeks from now on Mar. 12, 2011 - see you all @ www.michaeljuan.blogspot.com
The world of biotech is both exhilarating and rewarding. However, picking the appropriate companies to invest in can be challenging and at times depressing. In this blog, you will find information gathered from industry reports, analyst forecasts, and market trends, helping you to emerge from mindless speculation and make intelligent investment decisions! - Michael Juan
Saturday, February 26, 2011
URGENT: Change of URL - Mar. 12, 2011
Thank you all for the wonderful support and love for this biotech/pharma investment forum!!! It has been over a year since the opening of this blog and a lot of changes have happened in this year - notably the growth of my portfolio: 3600% growth since I made my first trade on Feb 25, 2010 (exactly a year ago).
Friday, February 25, 2011
Protalix (PLX) - failed attempt to get past FDA's stringent eyes
~~~ UPDATE: FDA rejects Protalix's Uplyso (taliglucerase alfa) ~~~
Protalix (PLX) announced today that the company has received a CRL (complete response letter) from the US drug regulator, which requested additional data from the switch-over trial and extended follow-up study. PLX shares dove down 25% in morning trading. However, those who purchased Mar $12.5 put options are enjoying their 50% profit on Ulypso's refusal.
Sunday, February 20, 2011
Syneron Medical Ltd. (ELOS) - post financial surge creates opportunity for put options play
Recent news
 Recent release of positive financial results caused Syneron's stock (ELOS) to surge 20.7% in a single trading session. This pushed the stock up much above it's 50- and 200-day moving average with unusually high trading volume. In addition, ELOS shot up to its peak share price in 2 years following company's reporting.
Recent release of positive financial results caused Syneron's stock (ELOS) to surge 20.7% in a single trading session. This pushed the stock up much above it's 50- and 200-day moving average with unusually high trading volume. In addition, ELOS shot up to its peak share price in 2 years following company's reporting. Why is this a great opportunity?
1. ELOS is temporarily out of line due to recent news (~20% surge in 1 day)
2. Historical data show steady share price movement (~20% change in 1 year)
3. Trading volume increased 10 times (~2,000,000) on the news but quickly dropped
4. Historical data show low trading volume (< 200,000)
5. Investors may want to take profit leading to a sell off in the next few weeks
Trading map
Purchase Mar $12.5 put options @ $0.20 in anticipation of a price correction (i.e. drop in share price) in the next few weeks.
About Syneron
Syneron Medical Ltd., together with its subsidiaries, engages in the research, development, marketing, and sale of aesthetic medical products worldwide. The company develops products based on its proprietary ELOS technology, which combines conducted radiofrequency energy, an electrical energy; and light or laser-based energy, an optical energy. Its products target a range of non-invasive aesthetic medical procedures, including hair removal, wrinkle reduction, and rejuvenation of the skin’s appearance through the treatment of superficial benign vascular and pigmented lesions, acne treatment, treatment of leg veins, treatment for the temporary reduction in the appearance of cellulite and thigh circumference, ablation and resurfacing of the skin, and invasive products for laser-assisted lipolysis, as well as develops dental laser devices. The company sells its products primarily to physicians and other practitioners, including plastic surgeons, aestheticians, medical spas, dentists, dermatologists, and cosmetic physicians. The company sells its products primarily to physicians and other practitioners, including plastic surgeons, aestheticians, medical spas, dentists, dermatologists, and cosmetic physicians. Syneron Medical Ltd. sells its products through a direct sales force in the United States and Canada, and through distributors in Europe, the Middle East, South Africa, the Asia-Pacific region, and South and Central America. The company was founded in 2000 and is headquartered in Yokneam Illit, Israel.
Thursday, February 17, 2011
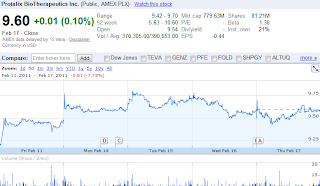
Protalix BioTherapeutics (PLX) - Taliglucerase Alfa FDA approval
Drug candidate: Taliglucerase Alfa
Indication: Gaucher disease
About Gaucher disease: Gaucher disease, the most prevalent lysosomal storage disorder, is caused by mutations in the human glucocerebrosidase gene (GCD), which have been mapped to chromosome 1 q21-q31, leading to reduced activity of the lysosomal enzyme glucocerebrosidase and thereby to the accumulation of substrate glucocerebroside (GlcCer) in the cells of the monocyte-macrophage system. This accumulation leads to the visceral manifestations of hepatosplenomegaly, anemia and thrombocytopenia, as well as to the skeletal features and less frequently also to lung involvement.
About Taliglucerase alfa: Taliglucerase alfa is a plant cell expressed recombinant glucocerebrosidase enzyme for the treatment of Gaucher disease. Expression of proteins in plant cell culture is highly efficient, does not require post-expression modification of the protein, and is not susceptible to contamination by agents such as viruses that are pathological to humans.
Note: Taliglucerase alfa was granted orphan drug designation by the U.S. Food and Drug Administration. A New Drug Application (NDA) for taliglucerase alfa has been accepted by the FDA and assigned a Prescription Drug User Fee Act (PDUFA) action date ofFebruary 25, 2011. Taliglucerase alfa is available to patients with Gaucher disease in the United States under an Expanded Access protocol as well as to patients in several member states of the European Union, Israel and other countries under Named Patient provisions.
Trading Map
Because Taliglucerase alfa's approval is widely expected, the upside is likely priced in already. Nevertheless, expect a $1-3 pop after FDA's announcement. The general consensus is that Protalix (PLX) could reach $10-12 with approval. With the PDUFA just around the corner (2/25), Mar $10 call options seem like a great buy. Look to sell these either BEFORE the catalyst or IMMEDIATELY after. After PDUFA, expect a quick sell off as traders scramble to sell on the news - look to snatch up Mar $12.5 put options. Good luck!
Sunday, February 13, 2011
Weekly preview - PLX, MNKD, AIS, NEOP, DCTH
Wednesday, February 2, 2011
Orexigen (OREX) - chubby gains from Orexigen's rejected diet pills
On Tues Feb. 1, Orexigen was faced with a train wreck as FDA rejected their high anticipated anti-obesity drug, Contrave. The stock (OREX) took an immediately hit, opening with a 75% haircut. Readers who took advantage of the advice given in several of my previous posts (1,2,3) enjoyed a large reward from their put options. Take Feb $9 put options as an example, the price spiked from $1.75 to $6.50 after Contrave's rejection by the FDA, representing an incredible 371% surge overnight (meanwhile call options, used to hedge the puts, of course slid from $1.70 to $.0.05)! If you had followed the call/put options combos suggested here, you would have enjoyed the following gains:
Tuesday, February 1, 2011
Orexigen (OREX) - FDA rejects Contrave, sending Orexigen back to the drawing board
~~~BREAKING NEWS~~~
FDA rejects Orexigen's Contrave
Orexigen's stock (OREX) crashed this morning as FDA rejected it's 'hopeful' diet drug, Contrave. The agency is worried about the heart risks associated with the drug and has requested for a trial with a large number of patients and longer duration. Enough said by the chart above and congrats to those anticipating the fall of OREX!
Subscribe to:
Comments (Atom)