MNKD and PSDV were mentioned in my previous blog post as year-end biotech stocks with a chance to pop prior their PDUFA (prescription drug user fee act) actions date, Dec. 29, 2010 for both.
The world of biotech is both exhilarating and rewarding. However, picking the appropriate companies to invest in can be challenging and at times depressing. In this blog, you will find information gathered from industry reports, analyst forecasts, and market trends, helping you to emerge from mindless speculation and make intelligent investment decisions! - Michael Juan
Tuesday, November 23, 2010
Friday, November 19, 2010
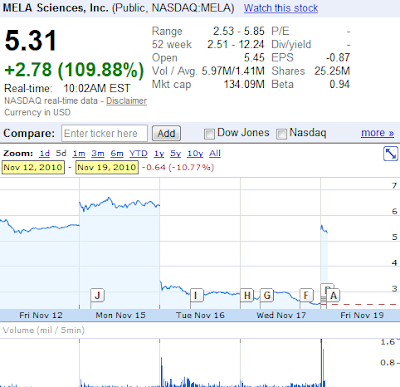
ALERT - Mela Sciences (MELA) bounced back more than 100% post FDA advisory panel
Mela Sciences (MELA), developer of handheld skin cancer detector, received marginal support from FDA's advisory panel yesterday, boosting its share price back up over 100%. As mentioned in my previous post, anything is possible; the biotech may in fact receive 'non-negative' recommendations from the panel. The vote came out 8-7. Eight out of 15 medical specialists on FDA's advisory committee voted FOR the passing of MELA's device.
MELA's shares more than doubled in pre-market trading. This may be a good opportunity to SHORT the stock as this overreaction is expected to wean in the next few weeks.
Thursday, November 18, 2010
Mela Sciences (MELA) - the ugly side of biotech
Mela Sciences (MELA), the Irvington, NY-based developer of handheld skin cancer detector, fell more than 55% in 3 trading sessions after FDA announced that the company's device might actually 'cause harm' to patients if approved. Adding to this, the biotech is under investigation for potentially violating federal securities law by 'misleading' investors with its reporting. It seems that ugly has turned uglier for Mela Sciences! Trading over $6 merely 48 hours ago, Mela Sciences has crashed to below $3 per share.
MELA will be facing FDA's advisory panel today to discuss the potential clearing of the device for marketing. While the general consensus is that the maker of MelaFind (handheld detector of skin cancer) will not receive positive recommendation from the panel of medical experts, it is never 100% sure what the outcome will be. A close tie may even relieve the biotech from the current doom. In any case, the path to device approval will no doubt be treacherous for Mela Sciences from this point on. Stay tuned for FDA's panel conclusion.
MELA will be facing FDA's advisory panel today to discuss the potential clearing of the device for marketing. While the general consensus is that the maker of MelaFind (handheld detector of skin cancer) will not receive positive recommendation from the panel of medical experts, it is never 100% sure what the outcome will be. A close tie may even relieve the biotech from the current doom. In any case, the path to device approval will no doubt be treacherous for Mela Sciences from this point on. Stay tuned for FDA's panel conclusion.
Monday, November 15, 2010
Biotech stocks to keep an eye on - year-end biotech stockwatch 2010
1. Valeant Pharmaceuticals International (VRX)
- Product candidate: Ezogabine (adjunct treatment of partial onset of seizures in adults)
- PDUFA: Nov. 30, 2010
2. pSivida Limited (PSDV)
- Product candidate: Iluvien (treatment of Diabetic Macular Edema)
- PDUFA: Dec. 29, 2010
- Product candidate: Afrezza (inhaled insulin)
- PDUFA: Dec. 29, 2010
Subscribe to:
Comments (Atom)