VIVUS Inc. (VVUS) is a biopharmaceutical company developing innovative, next-generation therapies to address unmet needs in obesity, diabetes and sexual health. The company's lead product in clinical development, Qnexa, has recently completed phase 3 clinical trials for the treatment of obesity. Qnexa is also in phase 2 clinical development for the treatment of type 2 diabetes
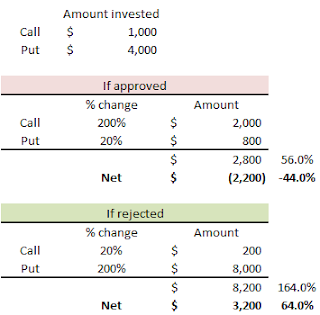
Catalyst
FDA Advisory Panel Meeting - July 15, 2010
PDUFA Date - October 28, 2010
 What is Qnexa?
What is Qnexa?
Qnexa is a once-daily, combination drug (phentermine 15 mg immediate-release + topiramate 92 mg controlled-release) in Phase 3 clinical development for weight loss in obese patients and improving blood sugar control in diabetics. Phentermine is the most widely prescribed prescription weight loss drug which is available in generic forms and has a database of 1.8 million patient years of usage.
Topiramate is better known as Topamax, which has 5.8 million patient years of use and was originally developed as a seizure drug and is also used for the prevention of migraine headaches. While both components of Qnexa will be available in generic forms, topiramate is not indicated for weight loss and the dosages being evaluated in clinical trials would make dosing difficult using the two drugs individually.
Vivus holds composition of matter and method of use patents for Qnexa as a combination treatment for obesity in the unique dosage forms that were investigated in several Phase III pivotal trials last year.
Clinical Trial Results
Stage: Phase III pivotal
Trial name: EQUIP (OB-302), CONQUER (OB-303)
Trial length: 56 weeks
Total # patients: > 3,750 across 93 sites
Summary: The EQUIP and CONQUER studies met all primary endpoints by demonstrating statistically significant weight loss with all three doses of Qnexa, as compared to placebo. Patients taking Qnexa also achieved significant improvements in cardiovascular and metabolic risk factors including blood pressure, lipid levels, and type 2 diabetes.
EQUIP
-> Wt loss: 37 lbs (14.7%) and 18 lbs with full-dose Qnexa and low-dose Qnexa, respectively vs. 6 lbs in the placebo group
-> 60% of the full-dose Qnexa patients who completed the study lost at least 10% of their baseline weight
-> 43% of the full-dose Qnexa patients who completed the study lost at least 15% of their baseline weight
-> Patients treated with full-dose Qnexa had significant improvements in blood pressure, triglycerides and cholesterol
CONQUER
-> Wt loss: 30 lbs and 24 lbs with full-dose Qnexa and mid-dose Qnexa,
respectively, vs. 6 lbs in the placebo group
-> Reduction in hemoglobin A1c levels of 0.6% from 7.3% at baseline as
compared to a reduction of 0.1% from 7.4% at baseline for the
placebo patients (p<0.0001) -> 39% of the full-dose Qnexa patients who completed the study lost at
least 15% of their baseline weight
Data extracted from VVUS press release